2013-2014 Translational Research Initiatives
Enhancing Opportunities for Industrial Engagement: Revisions to the Membership Agreement
Starting with its inception, ERC-RMB pursued a strategy for industrial member recruitment and retention consistent with that detailed in the ERC Best Practices Manual. Early results with this approach were promising but by year 3 member retention began to suffer and recruitment of new members became difficult. We have carefully examined potential causes for this outcome. It became clear that the difficulties we were facing were the result of a potential mismatch between best practices developed across ERC’s representing a variety of industry sectors, and the business realities in a specific industry sector; in this case biomedical technology. Moving products to market is this space is a time consuming and costly process, with a significant amount of technical and regulatory risk. The role of academia is often to reduce technical risk to the point where an innovator company can garner the financial support needed to conduct early prototype testing in animal models, with the aim of further reducing risk to the point that a large commercial partner in comfortable in investing in the product opportunity. This can occur as early as in early clinical testing, but often occurs only after a first regulatory approval, such as a CE Mark (see figure below). In this process, the needs of many customers must be considered, including third party payers, regulators, patients, clinicians, medical device companies and innovator companies. While the ERC must be sensitive to the needs of all of these parties, the primary customer for the ERC is the innovator company. ERC programs and policies should optimally support this sector. The reality for the typical innovator company is that they are cash poor, and more able to collaborate via sharing of resources. Larger companies may be more interested in relationships with ERC faculty and students than in technology that remains too risky for them to invest in. These realties must be taken into account in setting of membership policies.
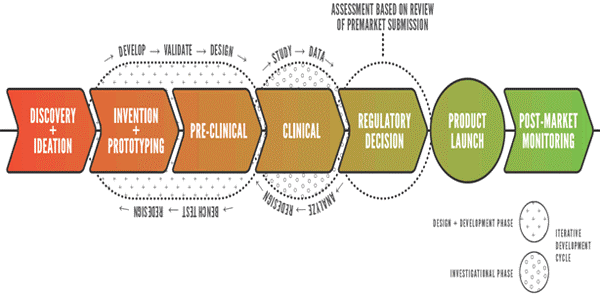
The membership agreement for RMB has been revised to reflect these and other realities. The bulleted points below were drivers in design of the new agreement:
- In any partnership, sharing of information is the highest value activity. For RMB, understanding the barriers to technology translation and the specific needs of potential commercial partners helps RMB focus its resources on those activities that have the greatest chance of attracting interest from potential partners.
- People are comfortable sharing information when they have developed a relationship based on trust.
- ERC-RMB membership is a commercial transaction. For an industrial partner to join the Industrial Affiliates Board, they must be able to justify a solid return on their investment in the ERC.
- Partners will come to us based on the excellence of our science and our ability to deliver on our promises (trust).
- When these factors are met, the possibility of moving from IAB membership to specific engagement exists.
- The ultimate goal is to achieve engagement of partners around a value proposition that works for both parties.
Given the above, the terms of the membership agreement were developed to help foster relationships, build trust, and provide value for the member that would help them further engage ERC researchers. The agreement provides a mechanism for faculty to demonstrate scientific excellence and the ability to deliver at low risk to the prospective partner.
Agreement highlights are bulleted below, a copy of the revised agreement is appended to this section of the annual report. :
- The financial barrier to membership has been significantly reduced. A single class of membership has been established, with annual dues set at $5000 ($2000 for companies with less than 50 employees)
- In-kind support can be used to offset membership fees. In-kind support is discounted at a rate of 50%.
- In order to help faculty build relationships with potential industrial partners, new IAB members that pay their membership fees in cash receive a first year right to engage the ERC researcher in a project of interest to them, valued at up to two times the membership fee. This feature allows the ERC researcher to demonstrate value to the IAB member; hopefully building a relationship that will lead to further engagement (joint writing of grants, research sponsorship, etc.) This one time offer also makes it easy for a prospective IAB member to justify membership fees to superiors in his organization.
- In years where support provided to the ERC by the IAB member exceeds $50,000 in value membership fees are waived. Support may come in a variety of forms, as detailed in the agreement.
- Student support is encouraged. In years where full scholarships are provided, IAB membership fees are waived, even if the $50,000 threshold above is not met. In cases such as this, an IAB member may be able to leverage recruiting and diversity monies in his organization to meet the contribution requirements for IAB membership, preserving funds for his lab while retaining the benefits of IAB membership.
- Access to IP is secured as a 6 month option to secure a royalty bearing, exclusive or non-exclusive license to IP developed during the term of active membership.
- Obligations of Industrial Affiliates include entering into a Non-Disclosure Agreement with the ERC, participation in the evaluation of ERC performance (SWOT)and reporting ERC progress, value to the site visit team (closed door session with industry).
- Industrial affiliates are also invited to provide guidance on ERC project selection, commercial relevance, and in general, provide support to project teams in project positioning and justification (commercial input to project QUAD Chart analysis).
Revisions to the membership agreement received approval of all parties in April, and are now being implemented. Commitments have been received from 6 parties, with paperwork in progress. Other memberships are being strategically pursued.
Industrial Engagement:
The ERC has implemented several mechanisms for industrial engagement, examples are provided below:
Embedded Researcher:
JetHot Inc. has funded Dr. Sudhir Nerrala in the ERC labs as a visiting scientist for the past several years. Dr. Neralla is an employee of Jet Hot, but spends 4 or more days a week working in the ERC labs in conjunction with Dr. Sergey Yarmolenko and Dr. Svitlana Fialkova. They work together on creation of high performance nanostructured coatings of interest to both Jet Hot and the ERC.
Support of Industrial Affiliates Technology:
The ERC has a longstanding relationship with nanoMag, a Thiomat Company. NanoMag has a proprietary process for producing magnesium alloys that may have sue in the medical of biodegradable medical devices, but lacks the in-house capability to evaluate for in-vivo evaluation of their materials. The ERC facilitated these studies through application of a combination of resources from NCAT (animal housing care, imaging and degradation analysis), PITT (local histology) and surgical assistance from Preclinical Translational Services which employs Vince Mendenhall, one of our scientific advisors. The program was funded under a supplemental grant to an STTR held by Thixomat. Successful completion of this study has led to increased investor interest for the Thixomat technology, and the award of a SECO grant to Thixomat to continue their work with the ERC as they refine alloy properties. The efforts have also expanded ERC capabilities and expertise, particularly in ex-vivo imaging assessment of biodegradation, hydrogen evolution and new bone growth. The developed technology has seeded a research collaboration with an investigator at Wake Forest University, grant applications are being prepared to further this work.
Translation of ERC Derived Technology: Licensing
In October of 2013, The ERC entered into an option to license agreement with inCube Labs. This represents a landmark event for the ERC, as it is our first tangible recognition of the commercial potential for ERC technologies and, more importantly, it engages the ERC in the translational process typically followed for medical devices; risk reduction through collaboration with an innovator company. inCube is a unique partner in that it has its own resources for technology assessment prior to creation of a startup organization. The ability to delay company formation until a proof of principle is achieved has resulted in a strong track record of success, one that is well appreciated by the investors needed to support the companies develop efforts and the ultimate large company acquirer of the developed technologies. Success risk reduction through this collaboration will make the ERC highly visible to all of the key players in the medical device space, most of whom have strong relationships with inCube and inCube spinoffs.
inCube’s option relates to specific ERC biodegradable metals technologies for orthopedic applications. Under the collaboration, inCube and the ERC will co-invest in advancing the technology prior to exercise of the option. Once exercised, the option will convert into a royalty bearing license. In the case of the licensing of a product based on ERC technology or the sale of a company stated by inCube based on ERC technology, the Universities will share substantially in the revenues to inCube. The ERC will continue to support the program through the bone plate and screw project, inCube’s contributions will include assistance in study design, patent and strategic marketing support, clinical target identification and product prototype design and conduct of a definitive animal study for the selected indication. inCube has also committed to hiring an ERC PhD graduate as a Post-Doctoral Associate at its San Antonio labs.
The partnership between the ERC and inCube has been joined by the Coulter Translational Partnership Program at PITT. This program provides funding to projects to help move them to an investment inflection point. The Coutler program has committed $125,000 of supplemental funding to support studies intended to produce the data required to move toward exercise of the option to license. This will initially take the form of a study to screen candidate alloys for physical and mechanical properties, rate of degradation and most importantly, develop methods for and provide an initial assessment of the pharmacokinetics of degradation and the local and systemic impact of the release of degradation products as the biometals degrade. The materials assessment portion of this study is underway.
Collaboration with Innovative Physicians.
Many innovative products in the medical device space are developed based on insights developed by clinical specialists. In some cases the clinician takes a leading role in realizing the product opportunity. Dr. Roy-Choudhury at the University of Cincinnati is such a clinician. He is a nephrologist with a strong interest in improving vascular access for hemodialysis patients. Access is optimally provided via creation of an aterio-venous fistula, which becomes a lifeline for patients in renal failure. Unfortunately, a substantial number of A-V fistulae fail to adequately mature post surgical creation. Dr. Roy-Choudhury recognized the for a degradable stent that could support the fistula maturation immediately post surgery and be resorbed within a 2 months of placement. He engaged the ERC in a project supported both by ERC funds and funding from his labs. This highly effective collaboration has resulted in our most advanced device project, one that is rapidly moving to the point where the proof of principle could be adequate to support licensing discussions. The interaction has also served to help the ERC better understand material behavior in high perfusion environments and led to the development of chemical etching technology for prototype stent manufacture.
Expansion of the Innovation Ecosystem.
Unlike inCube, most companies that may take on early translation of ERC technologies will be able to independently support their development efforts. They will not have internal resources, will have to raise funds to operate, and will likely operate virtually for some time. They will require access to resources and capabilities familiar with biodegradable medical devices on a contract basis. The ERC can optimally aid translation of its technologies by supplementing its research capabilities through build out of a consortium of companies and service providers that the innovator company will need to effectively design and de-risk prototype products. This access would not only provide comfort for the innovator company CEO, who would know that he is not left alone once he takes a license to ERC technology, but also to investors, who would know that they are investing in a company that has the requisite skill sets available to it to advance the technology to future value inflection points. The ERC has embarked on creating this consortium, whether through IAB membership or other associations. For example, OrthoKinetics brings expertise in mechanical and biomechanical testing and regulatory guidance, Fort Wayne Metals in metal processing and forging, Preclinical Translational Services in animal model section and study design, and the RJ Lee Group in particulate analysis and product quality assessments.
During the build out of these capabilities, we investigated ways to capitalize on the presence of the farm at North Carolina A&T State University to provide GLP compliant, long term medical device testing in large animal models. We realized that we would need a partner in this effort and initiated discussions with a company with a strong history of interacting with academic institutions and an interest in establishing a business presence in the Southeast. It soon became apparent to both parties that a collaboration between the company and the University could not only help enhance the ERC’s offerings, but also advance the educational and research and economic development missions of the University and significantly expand regional business opportunities for the company. A memorandum of understanding to support this collaboration has been signed by the company and University it is anticipated that the company will establish an office presence on the Gateway University Research Park campus and establish a pilot research facility by the end of the year. A formal announcement of the collaboration is expected in early 3Q.
ERC Technology Transfer
A. Inventions disclosed: 5
- Mark Schulz, “Nanovivo Robots to Change Interventional Medicine” (Date of inception: 3/2/2013), University of Cincinnati Invention Disclosure 113-063, Reported by: Mark Schulz
- Weifeng Li, Mark Schulz, “Spinning Superfiber from Long Carbon Nanotube Arrays” (Date of inception: 8/7/2013), University of Cincinnati Invention Disclosure 114-007, Reported by: Mark Schulz
- M. Schulz, B. Ruff, V. Shanov, Y. Song. “Additive Manufacturing using Carbon Nanotube Yarn and Ribbon Filament Materials,” University of Cincinnati Invention Disclosure UC 114-020 (Date of inception: 10/24/2013), Reported by: Vesselin Shanov
- M. Schulz, W. Li, D. Mast, S. Pixley, B. Ruff, V. Shanov, Z. Yin, Y. Zhang, “Nanovivo Robots to Change Interventional Medicine,” UC Invention Disclosure 113-063 (Date of inception: 3/15/2013), Reported by: Vesselin Shanov
- Y. Yun, Y. Koo and J. Sankar “Electrochemical Reduction of Carbon Dioxide at metal on functionalized highly-aligned CNT sheet electrode” North Carolina A&T State University Invention Disclosure EN0079 0114 (date of inception 1/24/2014), Reported by Y. Yun
B. Provisional patent application filed: 6
- M. Schulz, et al. “Smart Biomedical Implants Wiring-Up the Body to Live Longer, EFS ID: 15200276; U.S. Provisional Application Serial No. 61/779,356 (Date of inception: 3/13/2013), Reported by: Mark Schulz
- S. Woo, et al,.“Metallic and Extracellular Matric Bioscaffolds for Regeneration of the Anterior Cruciate Ligament” Provisional patent application No.: 61773435 (Date of inception: 3/6/2013), Reported by: Savio Woo
- Y. Yun, Y. Koo and J. Sankar, “CNT Sheets Derivatized with Transition Metals and Uses Thereof,” US Provisional Patent Application No: 61/936,129 (Date awarded: 2/5/2014), Reported by: Yeoheung Yun
- Yi Hong and W. Wagner “Biodegradable, Non-thromobogenic Elastomeric Polyurethanes,” US Provisional Patent No. 61771404 (Date of inception: 3/1/2013)
- P. Kumta et al., “Degradable Osteosensor (DOS): Novel Degradable CNT-based Impedimentric Biosensor for Bone Marker Detection”, Provisional Patent (Date of inception: 3/6/2013), Reported by: Prashant Kumta
- Yi Hong and W. Wagner “Biodegradable, Non-thromobogenic Elastomeric Polyurethanes,” US Provisional Patent No. 61/771,484 (Date of inception: 9/1/2013), Reported by: William Wagner
C. Full Patent Application: 2
- V. Shanov, P. Roy-Chaudhury, M. Schulz, Z. Yin, B. Campos-Naciff, Y. Wang, “Method for Making Magnesium Biodegradable Stent for Medical Implant Applications” Patent No: PCT/US 13/32374 (Date filed: 3/15/2013), Reported by: Vesselin Shanov
- Yi Hong, William R. Wagner, “Biodegradable, Non-thrombogenic Elastomeric Polyurethanes” Provisional Patent Application No, US 14/194,188 (Date of inception: 2/28/2014), Reported by: Sang-Ho Ye
D. Full Patents Awarded: 0

