ERC-RMB Accelerates Arteriovenous Fistula Stent Development and Commercialization with Support from the University of Cincinnati
May 2, 2014
Outcome/accomplishment: A product discovery team from a major medical device company visited the University of Cincinnati (UC). The team met with ERC leaders and PIs including Dr. Mark Schulz, Dr. Prabir Roy-Chaudhury, Dr. Vesselin Shanov and Dr. Zhangzhang Yin, and toured Dialysis Vascular Access Research Lab, NanoWorld Labs and Dialysis Clinic. The meeting was productive and both sides expressed interest in further collaboration. Dr. Dorothy Air, associate senior vice president for entrepreneurial affairs and technology commercialization, attended the meeting and conveyed that the University values technology commercialization and collaboration with industry. A similar meeting will be held with other potential industrial collaborators. These activities are an outcome of maturation of ERC biodegradable implant technology and ERC’s active efforts of seeking industry participation in research and commercialization. The Arteriovenous fistula (AVF) stent project is currently funded by the UC commercialization Accelerator, a program formed at the University of Cincinnati (UC) campus to create startup companies—ultimately leading to job creation—and to position promising early-stage research by UC faculty for commercialization.
With the help from Dan Kincaid, JD (Queen City Angels), an advisor assigned by the Accelerator program, Dr. Dorothy Air and ERC Industrial Liaison Officer Peter Seoane, Dr. Prabir Roy-Chaudhury and the AVF stent team started the process to form a company called “InoVasc” and has identified a trade name for the AVF stent (Flo-Fluent), put together a plan on setting up a company to apply for GCIC and STTR grants, and set up a long term plan whereby the team channel the academic findings and intellectual property from the PI’s laboratory (with permission) into the new company and ensure a product pipeline for InoVasc. The team filed a patent application (Method for Making Magnesium Biodegradable Stent for Medical Implant Applications”, International Application No. PCT/US 1332374).
|
Industrial people visits ERC-RMB and tours NanoWorld Lab |
Impact/benefits:
There are currently 100,000 AVFs placed yearly in the United States, all of whom would be potential candidates for the bMES because of the high rate of maturation failure. It is also anticipated that the AVF stent will expand the market for AVF placement by an additional 20,000 cases, since patients who are currently not a candidate for an AVF (small veins) could become suitable for this. A price of $1,500 (1.5k) for the AVF stent would result in a market potential of $180M per annum. And the potential cost savings to the health care system as a whole is estimated at $780M.
Started with $10K seed funding from the ERC, the AVF stent development has already undergone successful peer review and has received almost $600,000 (600k) internal and extramural funding in the form of University of Cincinnati CTSA (100k) and Accelerator (40k) grants and an NIH R-21 (427k).
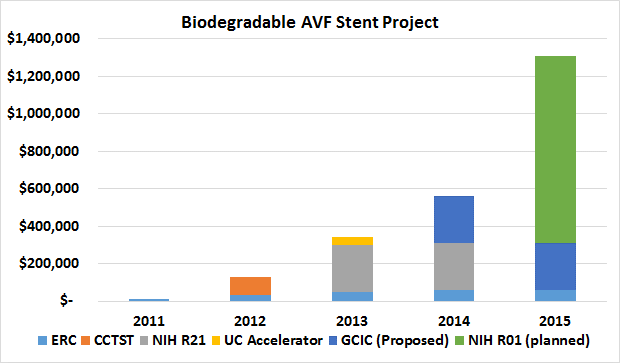
Sustainability of the AVF stent project. ERC funding of $60k/yr has brought a factor of 5 in additional funding in 2013; a possible factor of 8 (pending GCIC funding) funding in 2014; and a possible factor of >10 (pending a successful R01 grant) in 2015.
Explanation/ background:
As a newer project in the ERC-RMB, the AVF stent project was a fruit of ERC engineers’ dialogue with clinicians – Dr. Prabir Roy-Chaudhury and his colleagues. Dr. Roy-Chaudhury is a world-class expert in nephrology specializing in dialysis vascular access research.
|
|
Dialysis vascular access is clearly a “lifeline” for the 400,000+ patients on hemodialysis in the United States (over 1 million worldwide). Arteriovenous fistulae (AVFs) are the preferred mode of permanent dialysis vascular access because of better long-term survival and reduced infection risk as compared to dialysis grafts and tunneled dialysis catheters (TDC). This has resulted in a federal initiative to increase AVF prevalence, called “Fistula First”. Unfortunately, recent large studies have documented that only 40% of AVFs are suitable for hemodialysis at 4-5 months post surgery. Most AVFs fail to mature because of a peri-anastomotic venous segment stenosis likely due to a combination of an aggressive venous neointimal hyperplasia (VNH), together with a failure of outward remodeling. Despite the magnitude of the clinical problem, there are currently no effective therapies to prevent AVF maturation failure. Rather there is concern that the current standard of care (angioplasty and stent placement after the occurrence of stenosis) may actually reduce long term survival of the AVF due to vascular injury. At a pathogenetic level the three main causes for AVF maturation failure are (a) small veins (b) an abnormal non-laminar hemodynamic flow profiles (c) abnormal local endothelial function as a result of oxidative stress and inflammation in uremic patients; all of which result in a predisposition to VNH and impaired outward remodeling.
|
|
The introduction into clinical practice of a biodegradable maturation-enhancing stent in AVF which targets sequential pathways (venous segment diameter, hemodynamic profiles and local vascular wall biology) involved in AVF maturation failure could completely change current clinical paradigms for vascular access placement and maintenance. Thus it is likely that the pre-emptive placement of a bMES would (a) significantly reduce AVF maturation failure (b) avoid costly and often ineffective interventions (angioplasty) that are currently used liberally for AVF maturation failure (c) reduce the incidence and prevalence rates for TDC use in this patient population and so decrease the significant clinical morbidity and mortality associated with TDC complications (d) expand the patient population thought to be suitable for AVF placement (including pediatric patients), further reducing the incidence of PTFE grafts and TDCs (e) completely avoid the many negatives of a conventional stent with regard to restenosis and loss of future vascular access sites (f) allow for a significant commercialization opportunity in that the monetary benefits that would accrue to CMS (the single dominant payer for end-stage renal diseases) through fewer interventions for AVF maturation and fewer TDC complications would be many fold greater than the cost of the stent itself (particularly in current health care environment with its emphasis on bundling).
|
|
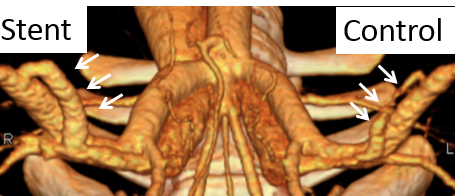
In the past three years, stents of different designs were manufactured and tested in a pig model. The AV fistula stents were designed within the ERC and currently are in the generation III design. Laser cutting, photochemical etching and braiding were the methods used to fabricate stents. The AVF stents were extensively tested in the pig model with AVFs created bilaterally in the groin. Clinical end points of flow and diameter were measured. In the limited number of pig experiments, the stent was found to increase blood flow and dilate fistula in general.
In addition, the team developed a novel approach to manufacture stents – photochemical etching, which is simple and has good accommodation for design changes and high throughput. The photochemical etching will generate no residual stress in the manufacturing process. A patent has been filed for the approach.