ERC-RMB Accelerates Arteriovenous Fistula Stent Development and Commercialization
June 3, 2015
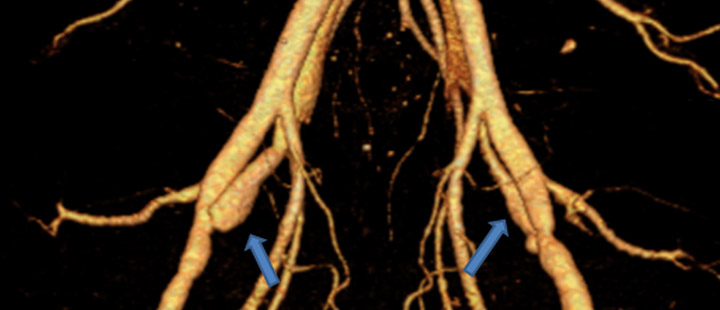
Outcome/accomplishment: A biodegradable drug-eluting stent that incorporated several ERC proprietary technologies was successfully tested in a pig model in Y7. In the one-month in vivo experiment, the biodegradable stent demonstrated the ability to dilate the arteriovenous fistula (AVF) (Figure 1) and increase the blood flow in the AVF with no observed negative response. The technologies incorporated in the AVF stents include an anti-proliferation polymer coating developed by the Dr. Wagner group, design and simulation by Dr. Yin, novel Mg alloy by Drs. Kumta and Xu, the pig AVF model by Dr. Roy-Chaudhury, and manufacturing technology by Drs. Shanov and Yin. During the in vivo testing, H2 sensors were used to measure the degradation of the stent by Dr. Heineman’s group. The ERC has established IP on most of these technologies.
|
Figure 1. Testing drug Eluting Stent in a pig model. The drug eluting stent was placed in the AV fistula, b) the bare stent in fistula (control), 3D CT Reconstruction of fistulae after 16 days. |
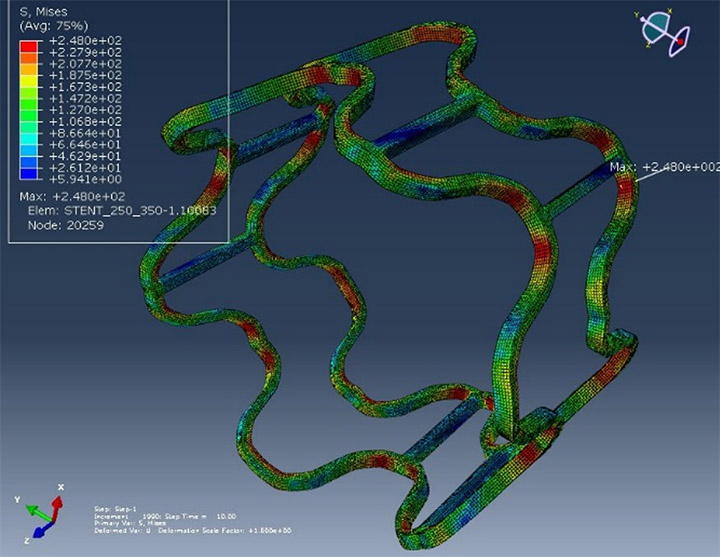
In Year 07 the team used computational modeling to assist in the stent design and to understand the deformation of stent during dilation. Figure 2 shows the strain and stress distribution in stents after dilation. Computational modeling was also used to compare ERC materials with commercial Mg alloys as stent manufacturing material. The ERC PL2 and PL4 materials demonstrated great potential as stent materials.
|
Figure 2. Modeling of Gen III AVF stent expansion: stress distribution after expansion. |
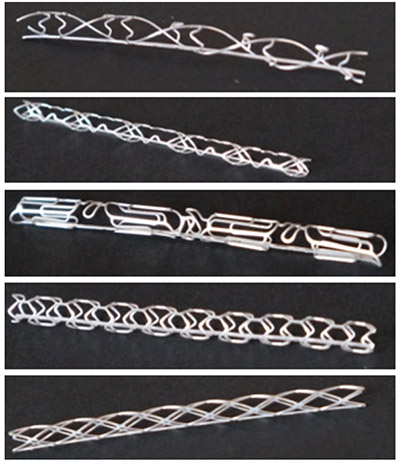
New stent designs that may optimize hemodynamics are in development. Dr. Shanov’s group manufactured five different stent designs using the chemical etching process (Figure 3). At the same time, the in vivo testing of Gen III stents continued to build up statistical proof of concept.
|
Figure. 3 Biodegradable Mg stents fabricated by photochemical etching in Y7 |
In Y7 more industries demonstrated interest in the AVF stent technology and collaboration is occurring in different formats including using an ERC member’s animal models for device testing.
With the help from Dan Kincaid, JD (Queen City Angels), an advisor assigned by the Accelerator program, Dr. Dorothy Air and ERC Industrial Liaison Officer Peter Seoane, Dr. Prabir Roy-Chaudhury and the AVF stent team started a company called “InoVasc” and has identified a trade name for the AVF stent (Flo-Fluent) and will be preparing commercialization grants such as GCIC and STTR.
Impact/benefits:
There are currently 100,000 AVFs placed yearly in the United States, all of which would be potential candidates for the bMES because of the high rate of maturation failure. It is also anticipated that the AVF stent will expand the market for AVF placement by an additional 20,000 cases, since patients who are currently not a candidate for an AVF (small veins) could become suitable for this. A price of $1,500 (1.5k) for the AVF stent would result in a market potential of $180 M per annum. The potential cost savings to the health care system as a whole is estimated at $780 M.
Explanation/ background:
As a newer project in the ERC-RMB, the AVF stent project was a fruit of ERC engineers’ dialogue with clinicians – Dr. Prabir Roy-Chaudhury and his colleagues. Dr. Roy-Chaudhury is a world-class expert in nephrology specializing in dialysis vascular access research.
Dialysis vascular access is clearly a “lifeline” for the 400,000+ patients on hemodialysis in the United States (over 1 million worldwide). Arteriovenous fistulae (AVFs) are the preferred mode of permanent dialysis vascular access because of better long-term survival and reduced infection risk as compared to dialysis grafts and tunneled dialysis catheters (TDC). This has resulted in a federal initiative to increase AVF prevalence, called “Fistula First”. Unfortunately, recent large studies have documented that only 40% of AVFs are suitable for hemodialysis at 4-5 months post -surgery. Most AVFs fail to mature because of a peri-anastomotic venous segment stenosis likely due to a combination of an aggressive venous neointimal hyperplasia (VNH), together with a failure of outward remodeling. Despite the magnitude of the clinical problem, there are currently no effective therapies to prevent AVF maturation failure. Rather there is concern that the current standard of care (angioplasty and stent placement in the occurrence of stenosis) may actually reduce long term survival of the AVF due to vascular injury. At a pathogenetic level the three main causes for AVF maturation failure are: (a) small veins; (b) abnormal non-laminar hemodynamic flow profiles; and (c) abnormal local endothelial function as a result of oxidative stress and inflammation in uremic patients; all of which result in a predisposition to VNH and impaired outward remodeling.